Automation News and Press Releases
Post your news here.
Tech Tip Siemens 3-point Stepper Control
September 5th, 2012
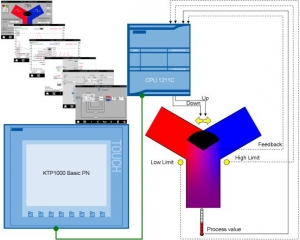
3-Point Stepper Control with SIMATIC S7-1200
For products: 6ES7211-1AD30-0XB0; 6ES7211-1AE31-0XB0; 6ES72
PROBLEM: The valve of a mixer tap shall be controlled according to a desired given temperature. A three-way mixing valve with valve actuator is used. The valve is digital controlled in both directions. This is referred to as a 3-point stepper control.
Varying the valve position can yield any mixing ratio of hot and cold entering water and so the water outlet temperature.
SOLUTION: For SIMATIC S7-1200 as of firmware V2.0, the development environment STEP 7 V11 provides the "PID_3Step" 3-point stepper control block.
Cross Company Pairs Hughes Safety Showers And Banner Wireless Monitors
August 30th, 2012
Cross Company’s Instrumentation Group, the premier provider of safety instrumentation in the Tennessee an Northern Alabama areas, announces the launch of a safety product offering unique to Cross customers. This offering will protect employees and satisfy OSHA auditors: Updated Hughes Safety Showers combined with Banner Wireless Monitors. This combination will automate reporting, allow for centralized status monitoring, and instant usage alerts within a 6 mile radius.
Knoxville, TN...
Kardex Remstar Helps Meet Executive Order 13423
August 20th, 2012
The year 2015 is fast approaching and federal agencies are required to be in full compliance with Executive Order 13423. Instated in 2007, Executive Order 13423 – Strengthening Federal Environmental, Energy and Transportation Management is all about sustainability for the future. By the year 2015, Federal Agencies are required to improve energy efficiency and utilize sustainable building design to reduce the current carbon footprint. With only three years left, federal agencies need to put...
Parker Eth Cylinder With High-speed Ball Screw Leads
August 9th, 2012
Among the Highest Force Density in its Class
Parker's new ETH electro thrust cylinder allows users to solve applications that require even higher speeds and accelerations than ever. It provides up to 5 times the force or up to 10 times the life (vs. comparable electric cylinders in the same ISO size). And its user-friendly design comes in a range of configurations to meet your specific requirements.
Typical ETH Applications include:
- test equipment and...
Dynamic Storage Protect Fda Regulated Labels
August 6th, 2012
The U.S. Food and Drug Administration (FDA) aims to protect and promote consumer health with regulations helping manufacturers and distributors improve production processes. The complexity of storing and retrieving packaging labels for drugs, vaccines or medical devices can vary greatly depending on the industry, FDA regulatory requirements and individual business models. Kardex Remstar dynamic storage solutions can makes these processes easier with security, inventory control, damage...
Kollmorgen Publishes Stepper Solutions Catalog
August 1st, 2012
July, 2012 – Kollmorgen’s Stepper Solutions Catalog details the features, benefits and specifications of Kollmorgen’s complete range of stepper motors and stepper drives, AC synchronous motors, XTRUE planetary gearheads and Optimized Solutions that result in rapid prototyping, shorter design cycles and getting machines to market faster.
Reply to this email to request a copy of the catalog.
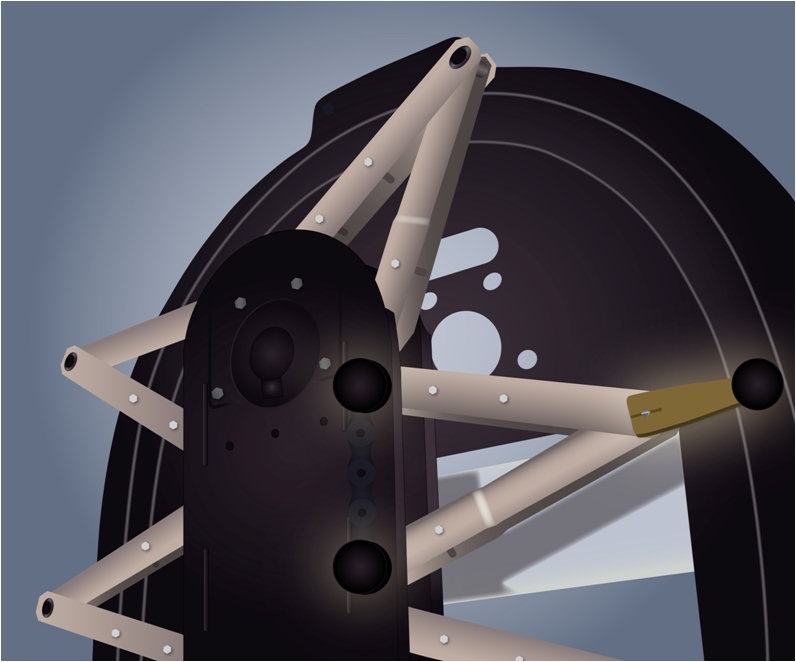
July 27th, 2012
Self-lubricating wheels on the scissor arm assemblies of Sapient Automation Avenger™ Vertical Carousels helps extend its operational life in storage and retrieval applications while reducing maintenance costs.
Generally, Vertical Carousels require periodic lubrication as part of a preventative maintenance routine to assure reliable operation. Self-lubricating scissor arm wheels reduce maintenance downtime and extend service life. This is particularly valuable during busy times when the...
Norco Low Power Product Series Based On Intel Ivy Bridge Processor
July 25th, 2012
NORCO, an Associate Member of the Intel® Intelligent Systems Alliance, released a series of new solutions based on the 3rd generation Intel® Core™ processor family and Intel® Q77 Express chipset. These solutions are ideal for industrial and embedded applications in Digital Signage, Retail, Transportation and Industrial automation systems.
Based on an innovative tri-gate transistor design and 22 nm process technology, the new 3rd-Generation Intel® Core™ ...
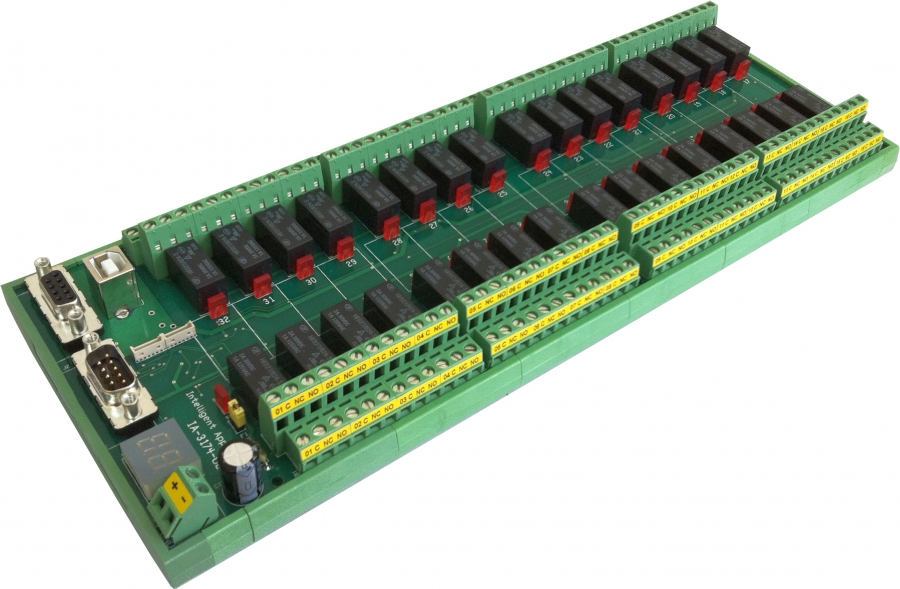
Industrial Isolated Usb Rs 232 Relay Controller Sold By Online Devices
July 24th, 2012
Online Devices has announced a new product by Intelligent-Appliance: IA-3174-U2. This is a new Relay I/O Controller with several new features released.
The IA-3174-U2 includes 32-ch DPDT relays with a complete wiring access of 6 wires per channel, several Operation modes that includes in addition to standard Random Access operation of 1 to 32 relays, a unique Single Relay operation or Dual Relays operation that might be used for Dual or Quad Pole multiplexing, that is firmed and well...
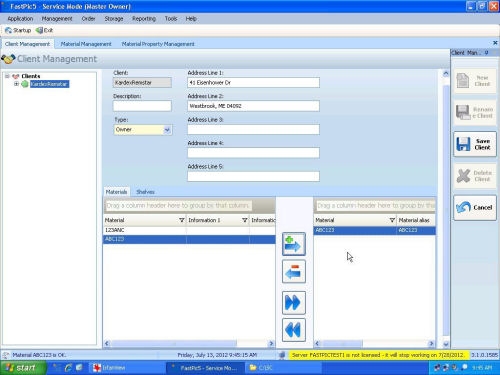
Fastpic Introduces Client Management
July 23rd, 2012
FastPic5 inventory management software introduces a new feature to help organizations segment inventory. The client management feature allows businesses to segment inventory by functional area or by client ownership. This organization tool allows for more security, visibility and ownership of goods.
With the new client management feature each user can be assigned to a specific category of inventory. This is particularly helpful for organizations that handle inventory for multiple...